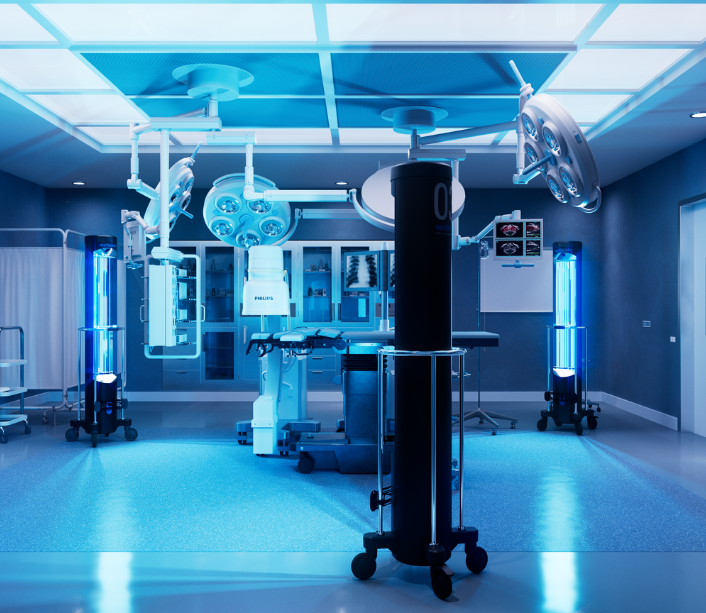
The SURFcare™ Program
Welcome to our white-glove concierge service. One point of contact for all of your customer service needs, allowing you to focus on what matters most: your patients.
Partner with usExceptional Client Service
Equipment Management and Service
Software and Firmware Updates
Cloud Database with Automated Reporting
Surfacide UVC microbial reduction: where best-in-class technology meets white-glove service to help provide patients the pinnacle of cleanliness and care.
Customer Service
A dedicated account manager will provide onsite visits and ongoing support, and wraparound support services will help you maximize and optimize technology, software, and services.

Equipment Maintenance
Receive hassle-free replacement or exchanges to minimize downtime of UVC microbial reduction equipment, and easily exchange a safety motion sensor, tablet and emitter for any non-working items. Plus, receive one total lamp system replacement during the life of the system.

Advanced Analytics
A newly designed dashboard with a robust selection of widgets can be customized to analyze data in a variety of ways, allowing you to easily and quickly access real-time data or create scheduled reports sent right to your inbox.

Maximize your investment with SURFcare
We offer the first year of SURFcare with every purchase at no additional cost. Hit the “easy button” for data management, turning real-time data into productive, tangible information, allowing you to continually improve your UVC microbial reduction program.
The latest from Surfacide

Transforming Healthcare with the Helios+ UV-C System: Supporting EVS Professionals with Adaptable, Empathetic Solutions
Read

Surfacide Unveils Helios®+ UV-C System: The First Whole Room Microbial Reduction Device to Receive 510(k) Clearance from the U.S. Food and Drug Administration
Read